Introduction
I am to investigate the effect on the rate of reaction which can be seen when I change first the concentration of acid used and then the types of acids used.
The acids which I will use are as follows:
- Hydrochloric Acid (HCl)
- Sulphuric Acid (H2SO4)
- Phosphoric Acid (H3PO4)
- Nitric Acid (HNO3)
- Methanoic Acid (HCO2H)
- Ethanoic Acid (CH3CO2H)
I will be investigating the effect on the initial rate of reaction. The other rates (e.g. after 2 minutes, or after a certain amount has been released) are dependant on this one, and may not be measurable in some cases. For example, if one was using a burette to collect the gas released: say an acid takes merely 1 minute to fill the burette (50 cm3) then one could not easily find it's rate of reaction after 2 minutes (it would either involve not taking the first 50 cm3s, or some messy change of burette half way). Furthmore, if one was to measure the rate of reaction after 30 cm3 of gas is released some acids may not release enough gas!
The methods are described next.
Methods
I will first test a few different acids, and then try some of them at different concentrations. Since my knowledge of acids tells me that the "active" part of acids is the Hydrogen (H+) ion, I will ensure that the concentration which I modify when changing concentrations is the concentration of the Hydrogen ions. Similarly, when trying different acids, it is the Hydrogen ions' concentration which I will endeavour to keep constant - even with acids containing more than one Hydrogen ion per molecule.
I will do each experiment twice, and then average the results. This is to reduce the error, and thus give me better data. I have two methods in mind, the first one and the one which I would like to do is the weight loss method, the second is the gas released collection method. Both are described below. The second method is the one recommended in books, and maybe there is a wise reason behind this, but nothing can be lost by trying the first method I describe, and there is all to gain (experience, in any case). The first method has the important advantage of being able to be connected to a data logging computer, thus the increasing accuracy of results and easing the data collection process.
Method 1
(Mass Change Method)
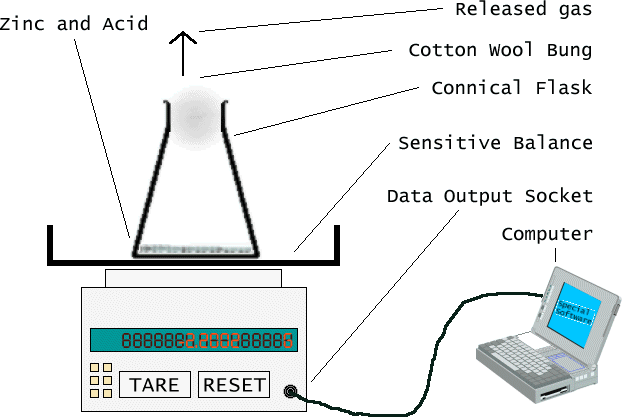
First, I will select an acid and a concentration, and then calculate the volumes needed (see below). Once I have made the acid at the correct concentration, and have cleaned the equipment (this is important since I do not want contamination) then I will (a) pour the acid into the conical flask, and (b) pour the acid and quickly stuff the bung into the flask while simulataneously telling the computer to start actively monitoring the balance's output data.
In case of problems with the computer, I would just write down the values manually, using a timer. To make my results more accurate, I would only take results when the mass changes. This means I effectively get a 1s timing resolution (the alternative is to take readings at fixed intervals, say 10s, but this means that the time at which the change occurred could be anywhere from at the point of recording to 9 seconds before, i.e. 10s timing resolution). Note it is only 1s timing resolution since my reflexes are only that good, by the time I have noticed the change, and then looked at the timer, at least a second will have gone by. For this reason I would not use the "lap time" facility and get a 10ms timing accuracy, since the error margin would still be around 1s (indeed more since I would have to hit the button) and thus the extra information gathered would be eventually discarded anyway (when allowing for errors).
The cotton wool bung is to reduce the risk of external contamination during the experiment, as well as to reduce the amount of "evaporating" or escaping acid (whenever any gas - eg, the Hydrogen released - leaves the liquid mixture at the bottom, some liquid - the acid - will come with it). Pressure will build up inside the conical flask and since the pressure will therefore be greater than outside the flask, the gas produced will escape (if it Hydrogen as I assume then it is lighter than air).
The computer method gives me much more accurate data, it works in milliseconds.
Method 2
(Gas Collection Method)
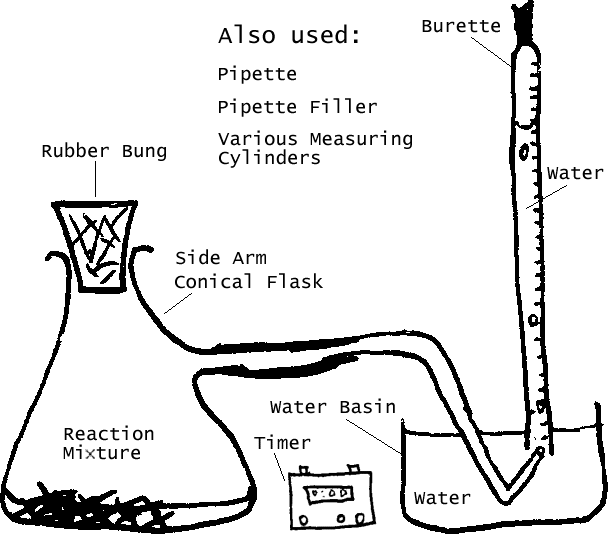
Underwater delivery reduces the risk of leaks. Before each test I will clean the conical flask, and refill the burette. As for the manual version of Method 1, I will only take down results when a bubble appears, giving me a greater accuracy than the fixed interval technique.
Calculating the masses needed
To find the concentrations, volumes and masses needed I used the following technique.
- Select the acid you will use. For example, H2SO4.
- Select the concentration of Hydrogen ions you want to use. To start with I will use 0.25 mol dm-3.
- I will keep the number of moles of Hydrogen ions constant at 0.015 mol
(this gives 60 cm3 of 0.25 mol dm-3 HCl - see sidebar - which is a reasonable and manageable amount).
The relationship between the volume of a solution (V), the number of moles of solute in solution (n) and concentration of the solute in the solution (c) is given by
V = n
CSubstituting,
0.015 mol
0.25 mol dm-3= 0.06 dm3 And of course 0.06 dm3 × 1000 = 60 cm3
- Figure out the number of Hydrogen ions present in one molecule of your chosen compound (in our case, 2).
- Divide the number of moles of H+ ions (0.015) by the number of Hydrogen ions per molecule of acid (in this case 2). This is the number of moles of acid you need (n), in this case 0.0075 mol.
- Divide the concentration of H+ ions you have chosen (in this case 0.25 mol dm-3) by the number of Hydrogen ions per molecule of acid (in this case 2). This is the concentration of acid you need (c), in this case 0.125 mol dm-3.
- Divide the number of moles of acid needed (n) by the concentration of acid needed (c) and you get the volume of acid needed. In this case, 0.0075 ÷ 0.125 = 0.06dm3. To get this value in cm3, merely multiply the value by 1000.
- To find the mass of Zinc needed (which is the same each time since the number of moles of Hydrogen ions is constant) multiply double the moles of H+ by the molar mass of Zinc (65.4 g mol-1). This gives you the minimum required mass of Zinc (0.5g to 1dp). I have used an excess of around 1.0g, thus the mass of Zinc I used is 1.5g each time. Note that it is double the moles of H+ because two Hydrogen ions are used to oxidise one atom of Zinc (Zn + 2H+ ® Zn2+).
Making Concentrations on Demand
Since 0.25 mol-1 is not in stock for most acids, I will have to make up the correct concentrations from 1 mol-1 or, sometimes, 0.5 mol-1 solutions. To make 0.25 mol-1 from 0.5 mol-1 is very simple, using a measuring cyclinder of sufficient size (eg 100 cm3 if you need 60 cm3 of acid) pour in half a measure of acid (eg 50 cm3) and half a measure of distilled water. To make more complicated solutions, eg 0.2 mol-1, I will use 1 mol-1 and then dilute it in a ratio of 1:5 (that is, 10 cm3 of acid for 50 cm3 of water). I will use distilled water since tap water has many impurities which would dirty my results in a most unpredictable and doubleplusungood fashion.
Prediction
Gas released
I predicted that the gas released during a reaction between an acid and Zinc is Hydrogen. Working on the basis of the Brønsted/Lowry theory of acids, I based this prediction on the following equation:
2HCl(aq) + 2Zn(s) ® H2(g) + 2ZnCl(aq)
or, ionically,
H+(aq) + e- ® ½H2(g)
This reaction definitely releases gas, preliminary tests confirmed that. They also confirmed it was Hydrogen (the gas popped when a lit splint was placed near it). Other gases may be present, depending on which acid I use, although I have no evidence that this is the case.
Effects on the Rate of Reaction
In the case of different acids, it is likely that acids which split into more stable ions in water (i.e. dissassociate better) will have greater rates of reactions. Of the acids I will use, I predict that HCl, H2SO4 and HNO3 will be the strongest and HCO2H will be the weakest.
I predict that the higher the concentration of acid, the higher the initital rate of reaction will be. Specifically, I believe it to be a straight forward linear relationship, that is, double [H+] º double the rate.
Changes
I unfortunately found that there was a wise reason behind the use of method 2. Method 1 doesn't work. (See the Notes on Experiments section below for more details). I was also wrong about there being nothing to lose in trying, as I lost two valuable hours in (unsuccessfully) trying to get the software to run. I therefore decided to employ method 2.
I also relunctantly decided to only do each experiment once. Including the time taken to calculate the amounts needed, prepare each acid, clean the equipment and actually do the test, I often took almost 45 minutes per set of data. It would have been nice to get a complete set of results twice, and consequently reduce the error, but I just didn't have the time.
During experiment 3 I noticed a leak, and thus changed my method of gas collection. Again, see the section below for more details.
Results
Presentation
I have noted a result at 0s, it indicates the starting reading on the burette (sometimes when aligning the water with the 50 cm3 mark, one goes too far. This has strictly no effect on the validity of the results). The "Vol Released" columns of the results tables take this into account.
Calculation of the Rate of Reaction
To find the rate of reaction we first need to find the concentration of acid at any time. When all the acid is used up, the mass, or volume, of H2 evolved is proportional to the concentration of acid at the start. Thus, the concentration of acid at time t is directly proportional to the total mass, or volume, of H2 at the end of the reaction minus the total mass or volume of gas released at that time t.
For a mono-hydric acid, the number of moles of acid (0.015) is equal to half the number of moles of H2 evolved. The molar mass of Hydrogen gas (2 g mol-2), times the total expected number of moles of Hydrogen evolved (0.015 ÷ 2 = 0.0075), gives you the mass of gas. Thus the total amount of gas released (mfinal) is 0.015g. Indeed, for acids with different numbers of H+ ions per acid molecule this value is still the same since in all my experiments I kept the number of ions constant.
Zn + 2HCl ® ZnCl2 + H2
Two moles of acid result in one mole of Hydrogen gas.
This weight allows the rate data for method 1 to be calculated. Method 2 requires us to convert this value into the volume of gas released. Treating Hydrogen as an Ideal Gas, and using the Ideal Gas Formula, we can calculate the total volume of Hydrogen released at room temperature.
The ideal gas formula is:
pV = nRT
- p (Pressure)
- = 1atm or 1.01x105 Pa
- V (Volume)
- is the unknown
- n (Quantity of Gas)
- = 0.0075 mol
- R (Universal Gas Constant)
- = 8.31J mol-1 K-1
- T (Temperature)
- = 293K (20 degrees Centigrade)
Reference: PHYSICS, A Textbook for Advanced Level Students, 2nd edition. Written by Tom Duncan. Published by John Murray (Publishers) Ltd. ISBN 0-7195-4336-3. Page 457.
Substituting, we get
| V | = | 0.0075 × 8.31 × 293 1.01 × 105 |
Which gives us a value for V of 1.808 × 10-4 m3 (to 4 significant figures). This is 0.1808 dm3 or 180 cm3.
Plotting Vfinal - Vt on the vertical axis against t on the horizontal axis gives us a plot of rACID = k[ACID]a where rACID is the rate of change of concentration of acid, k is the rate constant and a is the order of the reaction. Note that [Zn] is not in the rate equation: because Zinc is a solid it's concentration does not vary even though it's mass is decreasing. The units of the rate constant k are mol dm-3 s-1
Note that when plotting the [H+]/Time graphs, I used Vfinal - Vt for [H+], so the units are not in mol dm-3, as might be expected, but cm3. This is proportional to [H+].
Alternative Method of Calculation
The above method is quite long winded, and only gives the first tiny portion of the full graph since nowhere near the entire amount of H2 is evolved. This can take several days. The alternative is to simply graph Vol Released against Time, since the gradient of this is also proportional to the rate of reaction. The data plotted on these graphs should normally be roughly straight at the start and then taper off as time passes, but I found that this does not always occur in my results, the rate of reaction often appears to be quite contant for the first 10 minutes (which due to time constraints was all I measured). The initial rate is simply the gradient of the graph at the start of the reaction, going through the origin.
The reason that I do not always get a curve in the first 10 minutes is that if the reaction is only moderately fast, there is no appreciable decrease in the volume of acid present, thus the reaction rate will remain unscathed.
Notes on Experiments
Layout
On the results sheets, INVALID indicates that I made a mistake and that the data is not comparable to anything else (these results are included for completeness only) and NOT ENOUGH DATA indicates that I did not get enough data points to either plot a Vfinal - Vt against Time graph with any hope of getting a good value, or to get an initial rate from the equivalent Vol/Time graph.
Lines which are shaded in the results tables are also circled on the first set of Vol Released against Time graphs and are the values which I believe to be errorneous, either due to experimental error or because of the somewhat random nature of reactions. Maybe a lot of molecules were all lucky and hit themselves at the right angle all together, maybe the opposite happened.
Experiment 1
It took nearly 10 minutes for a change of 20mg, yet the reaction was quite active.
I concluded that the mass change method was not satisfactory due to two main (related) reasons: There is little to measure and the error margin is too great.
The low Molar Mass of the H2 (2g mol-1) means very little change in mass even when a relatively large volume of gas is released. This means that it is difficult to say if the fluctuations are entirely caused by the reaction or if in fact minor changes in the atmospheric pressure were also a major factor (i.e. the error margin is very big). Indeed, blowing on the very sensitive balance used can cause the reading to change by 0.05g easily, which is orders of magnitude greater than the 0.02g measured after around 10 minutes (2.5 times bigger, in fact).
A less sensitive balance could not be used since it would not measure the mass change. An even more sensitive balance would require a very controlled environment, where pressure changes, draughts, breaths, and other possible error inducing events could be eliminated, because at these accuracies the aformentioned sources of error become a very important. The school does not have such an environment.
I have, however, learnt that for future experiments this technique is one to consider if the gas released had a much higher molar mass, eg CO2, which has a molar mass of 44 g mol-1 (22 times bigger than Hydrogen's).
Experiment 2
I removed 1 worryingly low value from these results. This data is not very accurate, it jumps above and below the line of best fit frequently. It is the first experiment I did with method 2, so I was still getting my technique up to scratch!
Experiment 3
At 530 seconds, I noticed a leak. See experiment 5.
This does not seem very reactive, for a discussion of why Nitric Acid seems suprisingly quiet see experiment 8.
Experiment 4
This set of results is invalid due to calculation errors. I multiplied the concentration of H+ ions I wanted (0.25mol) by the number of H+ ions in H2SO4, instead of dividing, to get the concentration of acid which I needed. I then forgot to divide the number of moles of Hydrogen ions by the number of Hydrogen ions in the compound, to get the volume of acid I needed. The results sheets gives the values I actually used. Although valid in themselves, the results cannot be compared to anything else which I collected.
Although I could have rescaled the values, this would have been inappropriate since I am trying to test the accuracy of the equation in question! Furthermore, repeating the experiment was not unduly difficult. I repeated the calculations correctly and got more accurate data in experiment 5.
Experiment 5
Before carrying out this experiment I studied the leak situation, and found a remedy. I rotated the delivery tube around 45 degrees to the left, thus having the end of the tube firmly inside the burette and the other side of the delivery tube held tightly by the top of the basin.
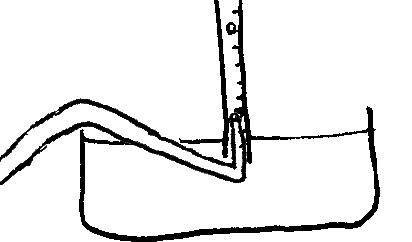
The first value is suspicious. The rest of the data was quite normal, with an odd but very small increase in the rate near the end of the data. The results from about 360s to 550s are suprisingly regular. This pattern (releasing 2 cm3s at a time) also appears later on, and during dicussions with colleagues it emerged that others had had similar experiences. This may be due to the delivery tube's shape.
Experiment 6
This data was more curved, as I originally expected all the results to be (since one would expect the rate to slow down after a lot of acid is used up). The last result is odd, it is as if the reaction sped up at the end!
Experiment 7
The data here is ok, apart from the first and third data points, which seem too high and too low respectively.
Experiment 8
Nothing happened! After researching into the matter, I discovered that NO2 and similar oxides of Nitrogen are released during the reaction. NO2 is very soluble in water, thus there is no or little collection.
Experiment 9
I used two times too much acid (another calculation error similar to experiment 4's). See Experiment 11 for a correct set of results for this attempt.
Experiment 10
Only the first point is odd here.
Experiment 11
It is difficult to know what to make of this data. At first it seems that it is a straight line but closer examination reveals that the reaction actually sped up after about 200 seconds. So the initial rate is slower than the rate at 300, 400 seconds.
Experiment 12
These results look very strange but really only the first point is off. It seems as if I started the experiment with low pressure inside the flask, and thus the volume released reading only went up after that had been overcome! This is possible, if irrelevant. I will merely discard point 1.
Experiment 14
Vineagar is not very reactive, as I predicted.
Experiment 15
Once again, the first and third data points are unexpected.
Experiment 16
This time the first and fourth data points are not as expected.
Experiment 17
As usual, the first data point does not follow the rest. In this case, the 8th is also out of the best fit line. This one is probably experimental error as opposed to the reaction getting started which is what I expect the first point's reason to be.
Experiment 18
The first and seventh are odd. The seventh is likely to be my fault, as I suggested for the 8th in Experiment 17.
Experiment 19
Prompted by a warning on the bottle of Zinc (to the effect of "Keep away from water" or "Keep in a dry place"), I decided to do a control test. Finding the biggest measuring cylinder I could, I poured 250 cm3 of water into a large side arm conical flask and added 5g of zinc. I then left it for a day. The reason for the initial reading being 48 cm3 and not 50 cm3 is that when I pushed the bung in the air got pushed out (for the other experiments the bung fitted in tighter, but this was a bigger flask).
However after 24 hours the volume had changed to 47.8, indicating a possible release of a negligable amount of gas. This is not important enough for the water diluting the acids to have any great effect (in any case it's presence was universal). There is not much evidence to suggest that the water was the cause either, although that is the most probable source. There way have been a leak, or someone may have touched it (it was left in a room in which classes were taught). Furthermore since 24 hours passed maybe the second day was warmer, hence expanding the air in the burette.
General
Of the 19 experiments, 12 were valid and had enough results to allow me to examine them. I have worked out the rates of reactions as read from the second set of Vol Released against Time graphs. The units of the rate are mol dm-3 s-1
| Acid | Conc mol dm-3 |
Initial Rate mol dm-3 s-1 |
|---|---|---|
| H3PO4 | 0.25 | 0.0667 |
| HCO2H | 0.25 | 0.0467 |
| H2SO4 | 0.2 | 0.0300 |
| H2SO4 | 0.25 | 0.0750 |
| H2SO4 | 0.4 | 0.0375 |
| H2SO4 | 0.6 | 0.0357 |
| H2SO4 | 0.8 | 0.0300 |
| HCl | 0.1 | 0.0500 |
| HCl | 0.2 | 0.0556 |
| HCl | 0.25 | 0.0455 |
| HCl | 0.3 | 0.0700 |
| HCl | 0.4 | 0.0500 |
Conclusions
Rates of Reactions
My results originally suprised me a lot. I am now firmly convinced that I should have done that second set of readings to take the average. The reason for my worries is the fact that the rate of reaction seems somewhat unreleated to the concentration of acid! Indeed for HCl the results are all over the place, and for H2SO4 the trend seems to suggest that the higher the concentration the slower the reaction.
In both (HCl and H2SO4) cases, the highest concentration I tested and the lowest concentration I tested gave the same initial rate of reaction. This is very strange. I did not believe this to start with, and so I checked it against the first set of graphs (vol released against time) but this is correct. Compare the graphs for HCl 0.1 mol dm-3 and HCl 0.4 mol dm-3 and you find they are very similar.
Explanations
Since my prediction does not match my results, either my prediction is wrong, I have misinterpreted my results or my results are invalid.
It would appear that the H2SO4 at 0.2 mol dm-3 results are incorrect. This would explain the odd pattern for the H2SO4 rates of reactions. If this is the case then (as far as H2SO4 goes anyway) the more concentrated the acid the slower it reacts. Although this seems nonsensical (to me, anyway) this is what the results are telling me. This is not a graph plotting error (I could have calculated the rate gradient incorrectly) since I checked my data.
What it means is that the more H+ ions one puts into a solution, the less they will react with the substances around them. This would also seem to explain why on one or two occasions the reaction seemed to speed up after 5 or 6 minutes (eg, experiments 6 and 11). I have known reactions to slow down when too much catalyst is put in, maybe the H+ ions are acting as their own catalyst. On the other hand, it could be the SO42- ion which is doing this. This seems more likely, although it goes against what I have learnt in the past concerning the Brønsted/Lowry theory of acids. However, the facts cannot be argued, and any theory must match the facts, so I have to assume that this final idea is correct. This could indeed indicate that the first result is not invalid, and merely an artifact of the SO42- ion acting as a catalyst.
HCl, on the other hand, is more logical. I believe that the incorrect results are those for 0.25M and 0.4M concentrations. If we remove these two then the trend is for an increase in rate as [HCl] increases. Much more logical, and what I predicted. Doubling the concentration certainly does not double the rate, but any accurate estimate as to the relationship is impossible since it is unclear what data is valid and which is invalid.
Different Acids
If HCl at 0.25 mol dm-3 is incorrect (it was my first attempt at method 2 so this may well be the case) then it would explain why HCl came up as the acid with the lowest rate (at 0.25M). Using the rate of HCl at 0.2M instead of 0.25M in the Rate/Acid puts it just below H3PO4, which makes sense although I thought Hydrochloric Acid was stronger than Phosphoric Acid. Of the four acids which gave me valid results, the order of rate of reaction is therefore (slowest) Methanioc, Hydrochloric, Phosphoric, Sulphuric (fastest). Nitric acid would be either top or one of the top three, but due to reasons discussed above, it does not give valid data.
Explanations
Sulphuric Acid disassociates probably 100% into the water, that is, the equilibrium
H2SO4(aq) ® 2H+(aq) + SO42-(aq)
is almost completely on the right hand side.
In the case of Phosphoric Acid,
H3PO4(aq) ® 3H+(aq) + PO43-(aq)
is the equilibrium and it is still generally on the right hand side but less so than H2SO4. And so on for HCl and HCO2H
Summary
It would appear that HCl gets stronger the more [H+] goes up. It would also appear that the SO42- ion in H2SO4 acts as a catalyst and actually slows down the reaction when it's presence reaches a certain point (namely around 0.3 mol dm-3).
Furthermore, the more stable the ions the acid splits into when in water, the stronger the acid is since the more dissasociated it becomes.
Notes on Presentation
Tables and Graphs
I apologise for the way compound formulae are presented on the results charts (eg, H2SO4 instead of H2SO4). This is due to the fact that the otherwise excellent Quattro Pro 6 spreadsheet product does not support discrete formatting within cells.
Attached Materials
There are one set of result tables and four sets of graphs included with this investigation. They are:
- Result tables for all 19 experiments.
- Vol Released against Time, including errorneous data
- Vol Released against Time, with initial rate best fit line
- Vfinal-Vt against Time
- The Rates of Reactions summaries
Bibliography
During the writing of this report I have consulted the 'A' Level Physics Book by Tom Duncan (2nd Edition), and
the Nuffield Advanced Science Chemistry Student's Book (3rd Edition), as well as the WebElements web site at
http://www.shef.ac.uk/chemistry/web-elements/.
I also used the following software: Novell Quattro Pro 6.01, HTML Notepad by Cranial Software, GNU Emacs 19.34.6 (i386-*-nt4.0) of Thu Sep 25 1997 on ESME, and Microsoft Internet Explorer 4.72.2106. My calculations were done using my TI-83.