Introduction
Some chemical elements have isotopes which emit radiation and as a result change into a different element. This process is know as radioactive decay or radioactivity and these isotopes are called radioisotopes.
Uses
Radioactivity can be used for:
- Dating old organic objects (Carbon Dating),
- Searching for blockages in the human body by eating food with traces of a radioactive material and then, using a detector, monitoring where the material goes (Tracers).
- The treatment of cancer
- Creating Electricity (Nuclear Reactors in power stations)
Background
Background radiation is a (low) level of radiation which is always present around us. Background radiation comes from a variety of sources, such as the following1a. The values in brackets are the number of mSv/year.
Natural Sources
- Cosmic Rays (0.3)
- Ground and Buildings (0.4)
- Food and Drink (0.37)
- Natural Radioactivity in the Air (0.8)
Sources due to Human Interaction
- Medical (0.25)
- Nuclear Weapons Testing (0.01)
- Air Travel, Luminous Watches, TV sets, etc... (0.01)
- Nuclear Power (0.002)
Typical Levels
In Melksham (south west England), the level of background radiation varies from around 0.28 s-1 on a good day to around 0.55 s-1 on a bad day.
Measuring
It is recommended that the background radiation be measured before any set of experiments, even on the same day, since it can vary considerably. A suitable means of measuring background consists of using the same apparatus as would be used with the source, but simply doing it without the source and for a longer time (since the count is much lower).
Detectors
Different radiation gets detected by different types of detectors. Most detectors simply indicate the arrival of energy. 2
Ionization Chamber
Detects alpha, beta.
Produces ionization
These are not very sensitive and work by charging two electrodes, one strongly positive the other strongly negative. By placing a galvonometer across the electrodes the ionisations can be detected as pulses of current.
Low energy gammas are also detected, but poorly.
Cloud and Similar Chambers
Detects Alpha, Beta, Gamma
Produces ionization
A charged particle travelling at speed leaves behind a trail of ions. If these occur in a region in which there is a vapour about to condense, or liquid about to boil, then the ions will encourage the formation of droplets and vapour bubbles respectively. Either will leave a trail to show the path of the particle, which may be photographed if the chamber is brightly lit from the side.
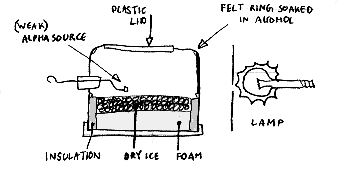
These principles are used in several devices.
The Wilson Cloud Chamber
In this, a sudden adiabatic expansion of the right amount cools water vapour so as to produce condensation on ions of either positive or negative charges.
The Diffusion Cloud Chamber
In this, a supersaturated alcohol vapour condenses. The pattern seen in the chamber is characteristic of the nature of the radiation which causes the radiation.
The Bubble Chamber
In a bubble chamber, charged particles leave trails of bubbles in liquid hydrogen. This has the advantage that any photograph will not be distorted by the expansion processes.
Geiger Müller Tube
Detects Alpha, Beta, Gamma
Produces ionization
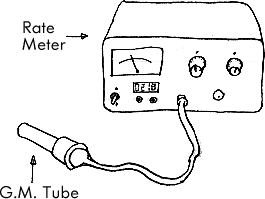
Typical Setup
 9
9Detail of Geiger Müller Tube
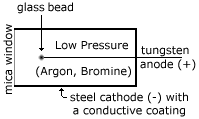
 4">
4"> Cross Section
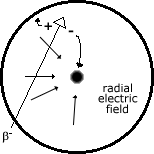
As some particle of ionising radiation (in the case of the cross section diagram, a b- particle) crosses the radial field caused by the p.d. of 500-1000 V between the central anode and surrounding cathode, it ionises the gas molecules. The gas molecules I am talking about are Argon and Bromine at low pressure in the tube. The positive ion created in this ionisation goes towards the negative cathode, and slows down as it does so (since it is a radial field, the force is denser at the center, and relatively spread out at the periphery). The negative electron 'produced' accelerates towards the anode, causing more and more ionisation.
The resulting avalanche causes a measurable blip. This is called charge/gas amplification. It should be noted that the speed of the electrons is actually not the cause of the blip, it's only the cause of the extra ionisation. The speed does not cause the current peak. The sheer number of electrons does.
The longer the pulse, the longer the dead-time. If the positive ion did some pair production too (as it would if it were sped up) then it would take time for the negative electrons produce to get back the anode. This would increase the dead-time, or even double the pulse, causing two blips instead of one!
Typical dead times are 0.1 to 1.0 milliseconds.
The glass bead is to prevent a very dense concentration from building up at the tip, which would cause the Geiger Müller tube to self-discharge!!!
Spark Counter

Detects Alpha
Produces ionization
This uses the ionization produced by particles to trigger a spark discharge in a region where air is close to breakdown in a strong electric field.
Scintillation Counter
Detects Alpha (with photomultiplier), Beta, Gamma
Produces flurescence in a phosphor
A scintillation counter consists of an appropriate phosphor combined with a photomultiplier tube, which enables the weak flashes of produced by beta particles and gamma rays to be detected.
Photomultiplier
The light output from the window is directed into a photomultiplier. This is a sealed evacuated glass tube which contains a series of electrodes. The inner surface of the front of the photomultiplier is coated with a photoemissive material which absorbs the photons and re-emits the energy in the form of electrons, as in the photoelectric effect.
These electrons are then accelerated by a DC voltage to the first electrode. Each incident electron cause the emission of two or three electrons which are accelerated in turn to the next electrode where the process is repeated. The last electrode produces the final output pulse which is now a cascade of electrons. A typical photomultiplier has a gain of between 104 and 106 and the potential difference between successive electrodes is about 100 Volts.
The impact of radiation on a suitable material (which can be matched to the radiation) causes the emission of a minute flash of light - called a scintillation. In the spinthariscope alpha particles cause zinc sulphide (a phosphor) to scintillate. A single crystal of sodium iodide is used for counting gamma rays.
The advantages of such scintillation detectors are:
- The energies of the particles detected can be measured.
- They can deal with very high count rates and very short pulse durations (in the order of 10-9 s).
- Their efficiency in counting gamma rays is almost 100%.
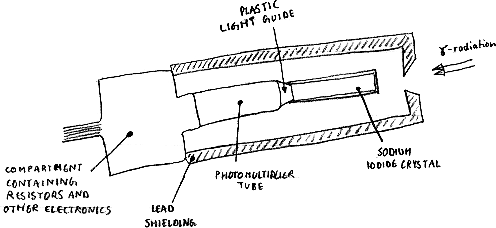
Some materials, when they absorb ionising radiation, produce seconday electrons by the photoelectric, Compton scattering and pair production proceses that generate photons of light. These materials are called scintillators. The light is emitted in a short pulse or flash and the amplitude absorbed so that the level of ionising radiation absorbed. If a meterial of a high atomic number os used, the sensitivity is increased.
The most common material used as a scintillator is sodium iodide which is activated by the addition of a small amount of thallium. Sodium iodide produces a very large amount of light compared to other scintillators. It takes about 10 nanoseconds for the light to reach it's maximum after the ionising radiation is absorbed. This is it's dead-time.
The scintillator is contained in a thin aluminium can, to prevent light entering the outside. The can is sealed to keep moisture out because iodide is hygroscopic, that is it absorbs water from the atmosphere. The inside of the can is coated with powdered titanium dioxide, which acts as a reflector to direct all the light from the scintillator through a quartz or plexiglass window in the rear of the can.
The main use of scintillation detectors is in nuclear medicine, where the gamma radiation comes from a radioactive material inside the body. The gamma rays pass through tissue easily and are detected outside the body by a scintillation detector in a device such as a rectilinear scanner or gamma camera.
Photographic Film Badge
Detects Mainly gamma, since the others are not likely to get close enough due to their range. However, in theory Alpha and Beta are quite able to produce tracks.
Produces exposure of photographic emulsion
A primary ion colliding with an emulsion gives a line of silver grains on processing. This enables a well-defined permanent record to be made, which can even be made three dimensional by stacking the plates. The emulsion used for detecting radiation has a high density of silver halide which results in the particle tracks being short.
Tracers
In medicine, doctors often introduce various radioisotopes or labelled compounds into patients' bodies 4. This is done in order to collect information about parts of the body such as blood, urine or a particular organ. Once administered, the tracers are followed (traced) by use of radiation detectors (discussed above)
Tracers are typically introduced in small doses, so that the system under investigation is not changed, and are typically samples of high specific activity, so that the small doses can still be detected. In addition to this, the tracer must behave like the tracee, so that it accurately reflects the body's normal behaviour.
If the tracer is a radioisotope, the small difference in mass between the tracer and the tracee is generally insignificant (e.g. 24Na vs. 23Na), but if a labelled compound is used the effect can be much greater.
Gamma emitting radioisotopes are preferred, since they can be detected externally. Beta emitters are occasionaly used, but these sometimes require the use of internal miniaturised Geiger Counters. Furthermore, beta emitters lead to higher absorbed rates since they are more heavily ionising.
Technetium
99mTc is the single most important radionuclide used at present. It's exploitation during the 1970s has been largely responsible for the rapid growth of nuclear medicine imaging techniques.
It has many advantages.
- Short half life in the region of 6 hours (will not linger for many days slowly ionizing remote parts of the body).
- Emits gamma rays exclusively (resulting in less damage than the more ionizing alpha and beta emitters).
- Easily detected by gamma cameras (140 keV - high enough to reduce scatter yet still within bounds of optimum detector efficiency)
- Readily produced on-site.
Typical Applications of Tracers
| Study | Tracers | Use, Comments |
|---|---|---|
| Body Composition | 3H, 82Br, 24Na, 42K | Volumes of various body fluids and estimates of quantities of (for example) sodium, chlorine or potassium present in the body. |
| Bone | 45Ca, 47Ca, 85Sr, 99mTc | Calcium absorption, bone mineral metabolism and localisation of bone disease investigated. |
| Blood | 131I, 132I, 125I, 51Cr, 32P | Estimates of various volumes, for example plasma, red blood cells, total blood, limb blood. Internal bleeding sites located using radioactive endoradiosonde. |
| Thyroid | 131I, 132I, 125I, 123I, 99mTc | Assessment of thyroid function. 132I (smaller doses) useful for pregnant women and children. |
| Liver | 198Au, 131I, 32P, 99mTc | Liver disease and disorders of hepatic circulation diagnosed. |
| Heart and Lungs | 131I, 133Xe, 99mTc | Cardiac output, blood volume and circulation times evaluated. Labelled gases used in respiration studies. |
| Tumours | 32P, 99mTc, 131I | Detection, localisation and differential diagnosis of tumours. |
| Therapy | 131I, 32P | Radioiodine used in thyroid treatment and radioactive phosphorus in certain haematological conditions. |
Glossary and Notes
- Ion
- A charged atom.
- Isotopes
- Atoms with the same number of protons (and electrons) but different masses.
- Labelled Compounds
- Organic compounds into which radioactive atoms have been artificially incorporated. 4
- Nucleons
- Protons and Neutrons.
- Nuclide
- An atom with a particular proton/neutron combination.
- Sievert
- The sievert is a measure of the biological effectiveness of radiation. For X-rays and gamma rays, 1Sv coresponds to the deposition of 1 joule of energy per kilogram of material. Strictly speaking, this unit should only be used when discussing delayed effects. The appropriate unit for early effects is the gray. 1
- Tracee
- The tracee is the substance being investigated by a tracer. 4
Expressions
A standard way of writing out the reaction invloved in a nuclear reaction is 3
initial nuclide (incoming particles, outgoing particles) final nuclide
Bibliography
- 1: The effects and control of radiation
- Second Edition. This is British Propaganda published by the
UK Atomic Energy Authority.
This booklet contains a number of diagrams claiming to be from other
sources, of which I have quoted one:
- 1a: Sources of radiation in the UK, from the National Radiological Protection Board.
- 2: Essential Principles of Physics
- Whelang & Hodgson. Second Edition. Published by BPCC Hazell Books. ISBN 0-7195-4566-8
- 3: Physics, a textbook for Advanced Level Students
- Tom Duncan. Second Edition. Published by John Murray (Publishers) Ltd. ISBN 0-7195-4336-3
- 4: Medical Physics
- Jean A. Pope Second Edition. Published by Heinemann Educational Books Ltd. ISBN 0-435-68682-8